
Recent news

CLIMATE
2024.04.18
Increased CO2 emissions from world’s tundra surprise researchers

Mental health
2024.04.17
Fluctuating coffee prices put mental pressure on Vietnamese farmers

Quantum Data
2024.04.15
Internet can achieve quantum speed with light saved as sound
species
2024.04.12
Iconic savanna mammals face genetic problems due to fences and roads

Sonning Prize
2024.04.11
After stroke: Marina Abramović to receive the Sonning Prize

poverty
2024.04.11
Economist: Tens of billions of dollars in forest products are being overlooked
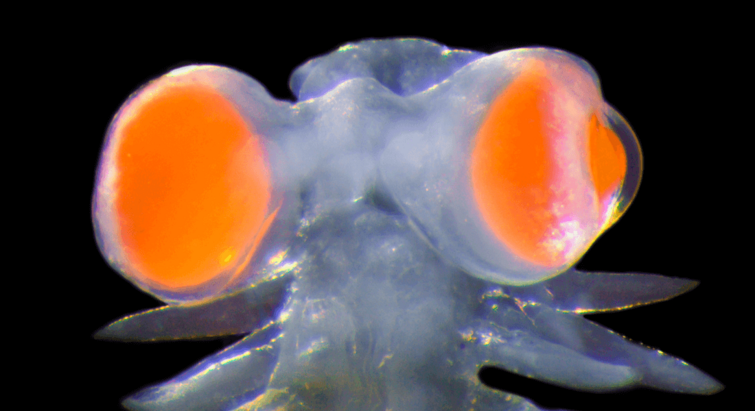
Marine Biology
2024.04.09
Mediterranean marine worm has developed eyes “as big as millstones"

Diversified farming
2024.04.05
Major study reports that people and environment both benefit from diversified farming, while bottom lines also thrive
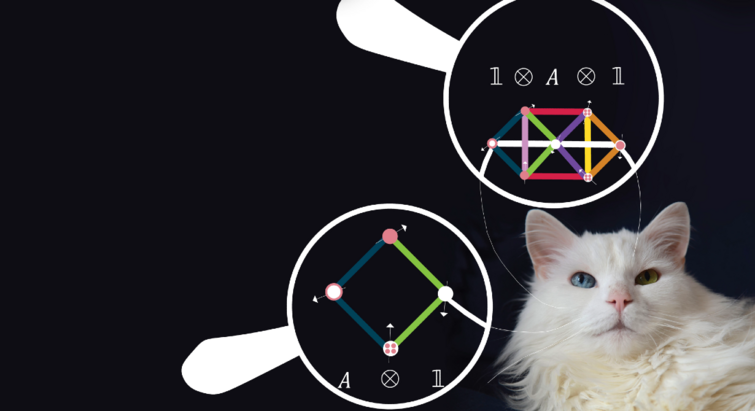
Quantum Particles
2024.04.04
“It’s ultimately about predicting everything” – theory could be a map to hunted quantum materials

artificial intelligence
2024.04.03
Computer scientists show the way: AI models need not be SO power hungry

Green data
2024.03.26
Health data storage has a climate cost. In the future data may be stored in DNA

Green medicine
2024.03.26

